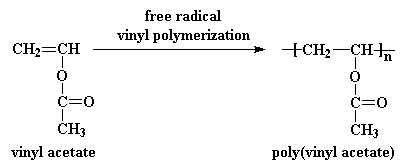
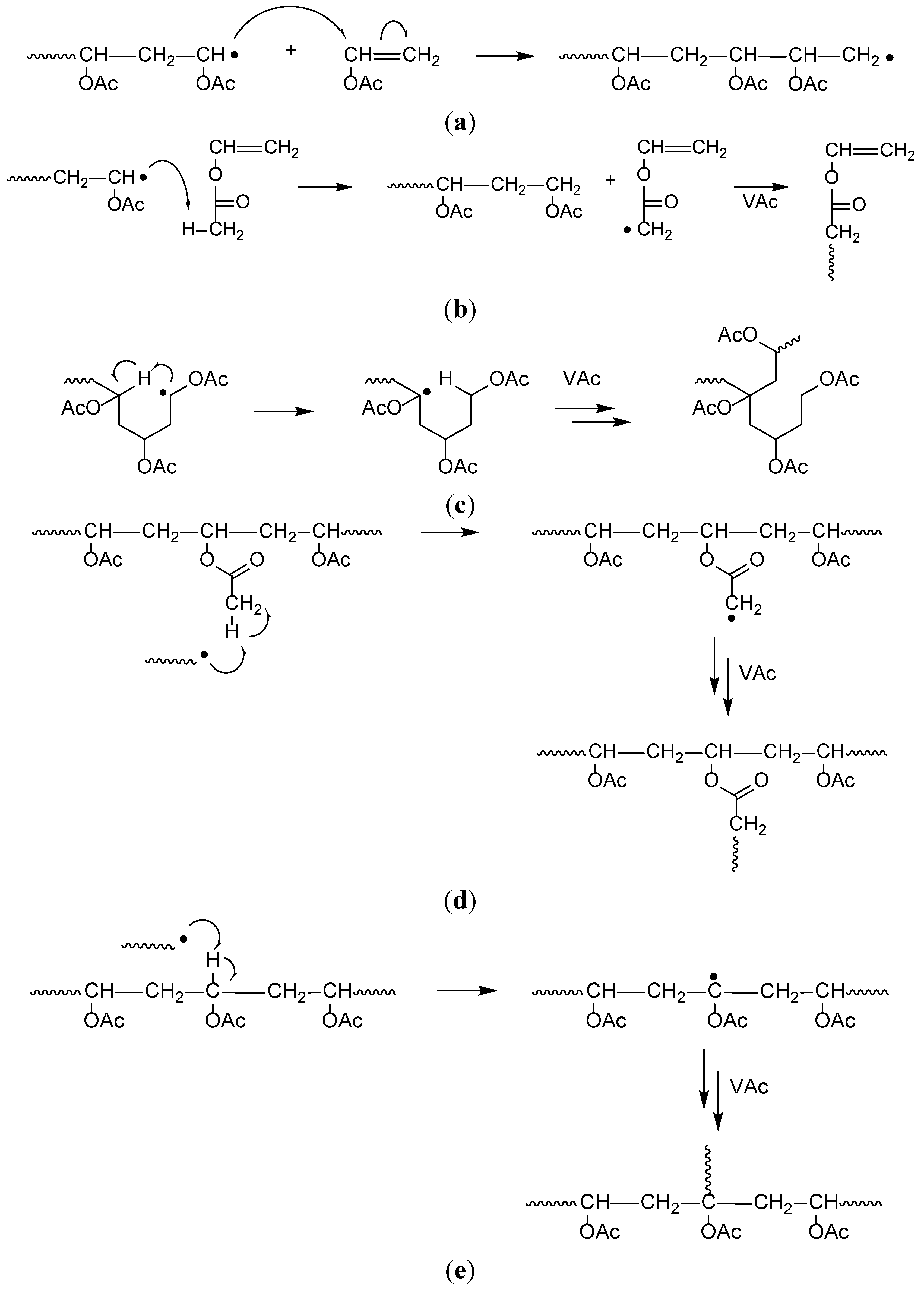
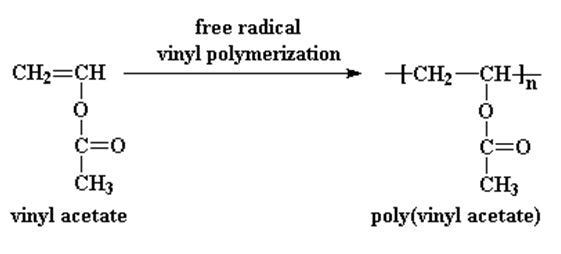
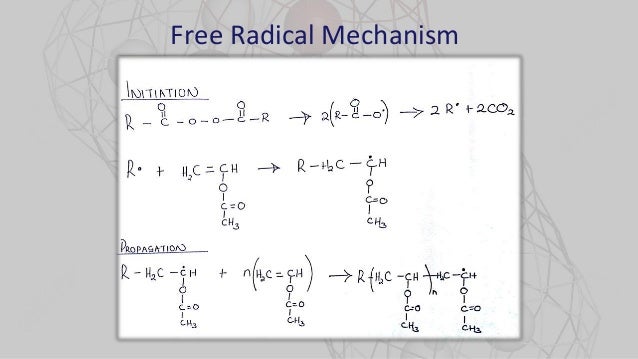
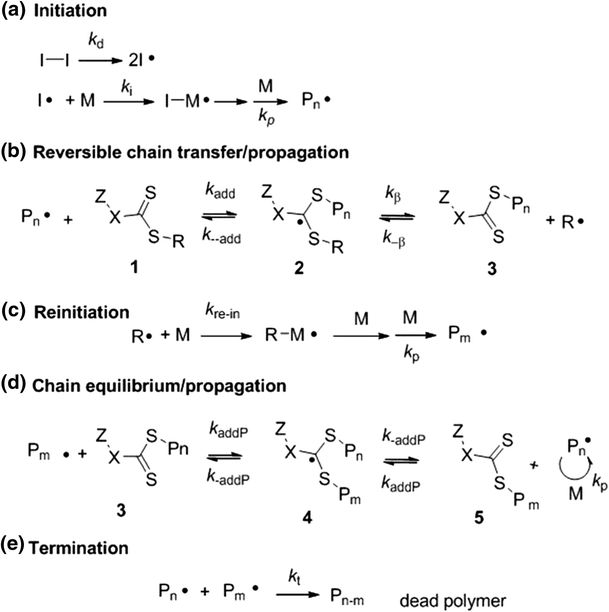
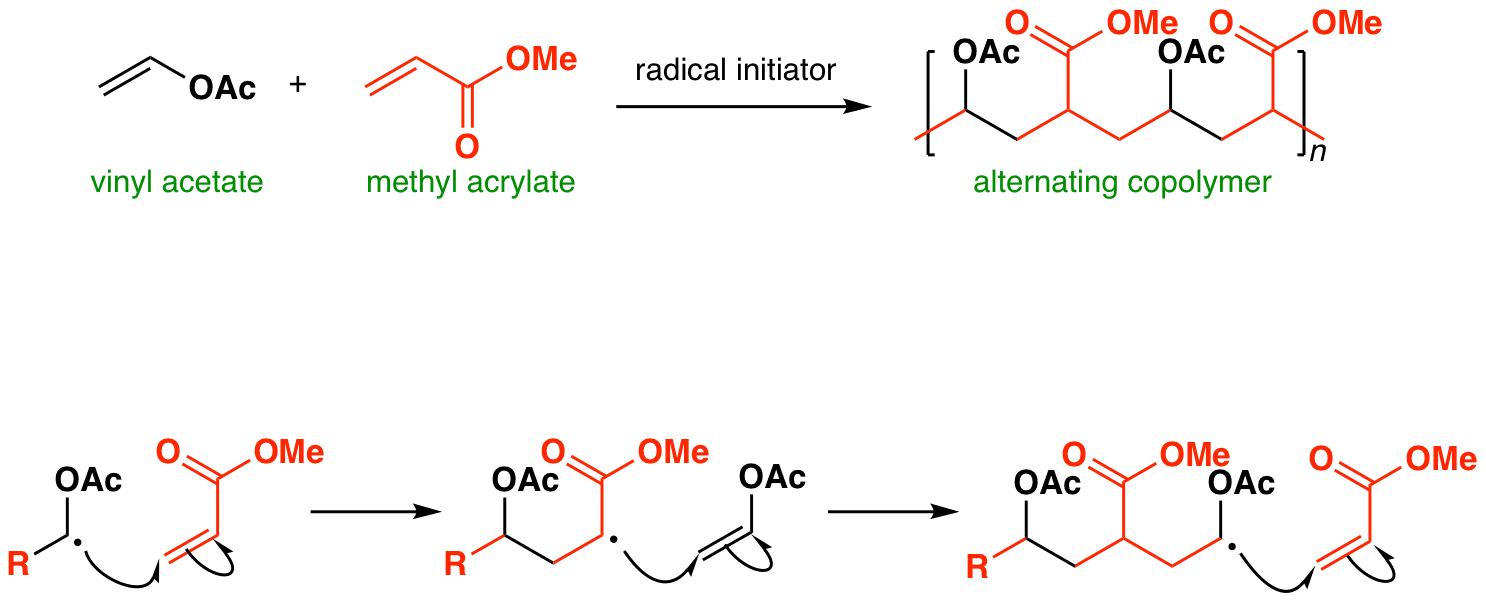
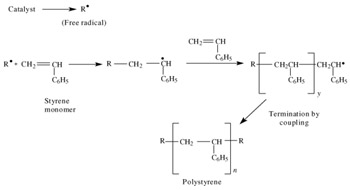
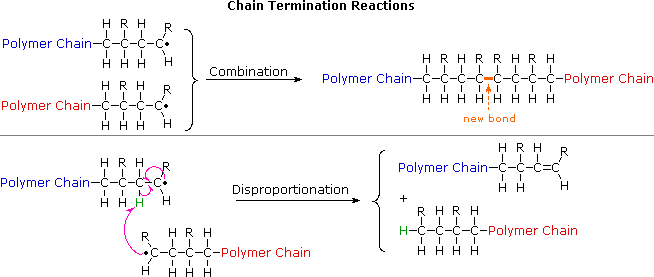
Polymerization Of Vinyl Acetate Mechanism
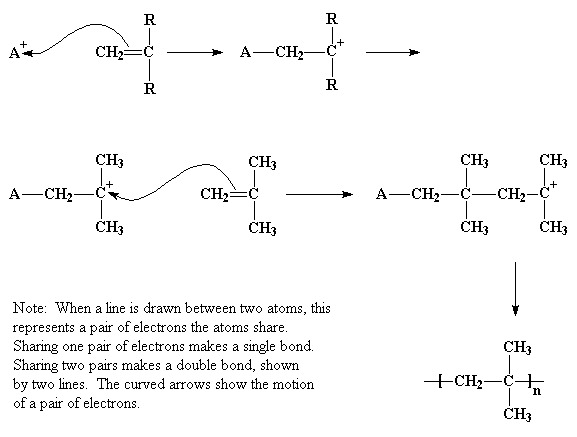
By electron microscopy the.
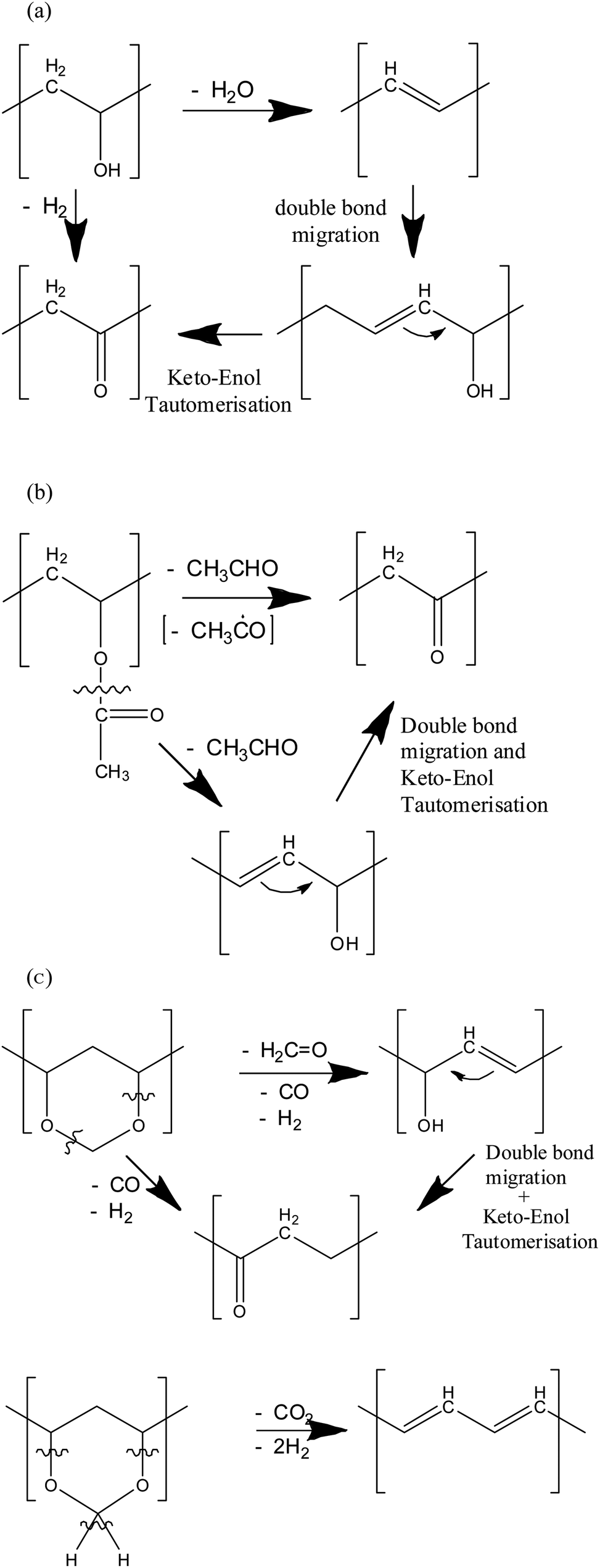
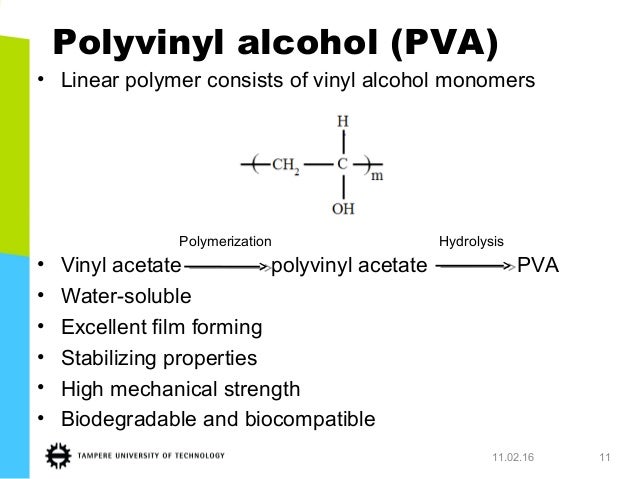
Polymerization of vinyl acetate mechanism. The degree of polymerization was. Pemberton napier college colinton road edinburgh eh10 5dt uk and. The initiator was azobis isobutyron1trile. Kinetic studies and polymerization mechanism d.
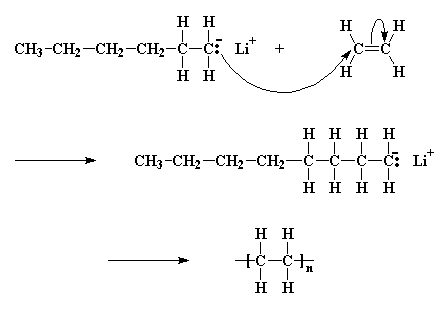
Zhigang xue rinaldo poli investigation of bis acetylacetonato iron ii as a moderator for the radical polymerization of vinyl acetate progress in controlled radical polymerization. That is from small molecules containing carbon carbon double bonds. Polymers made by free radical polymerization include polystyrene poly methyl methacrylate poly vinyl acetate and branched polyethylene. Vinyl acetate vac is one of the most challenging monomers to work with in preparing polymers with controlled architec tures.
It is used to make polymers from vinyl monomers. Polymerizations were carried out at 50 0 c 1 and one atmosphere pressure in a 0 5 liter four neck flask. Polymerization of vinyl acetate using visible radiation and a dye reducing agent sensitizer. Radical polymerization and increases its conversion rate to 95 with a relatively low molecular weight distri bution.
The bulk polymerization of vinyl acetate in storage vessels occurs spontaneously under constant temperature conditions due to a chemical acceleration phenomenon related to the free radical nature of vinyl acetate chain polymerization. Both the aqueous phase. Several aspects of this system have been clarified including the induced decomposition of persulfate retardation by oxygen and entry by and. Mechanisms and techniques 10 1021 bk 2012.
The mechanism of growth of latex particles in the emulsion polymerization of vinyl acetate using a polymerizable surfactant sodium dodecyl allyl sulfosuccinate trem lf 40. This work investigates the kinetics of the emulsion polymerization of vinyl acetate. Polymerization of vinyl acetate. Contrary to what has been reported.